FREE SHIPPING!* Flowflex™ Covid-19 Antigen Home Test, 50 Tests ($4.30 per test)
$215.00
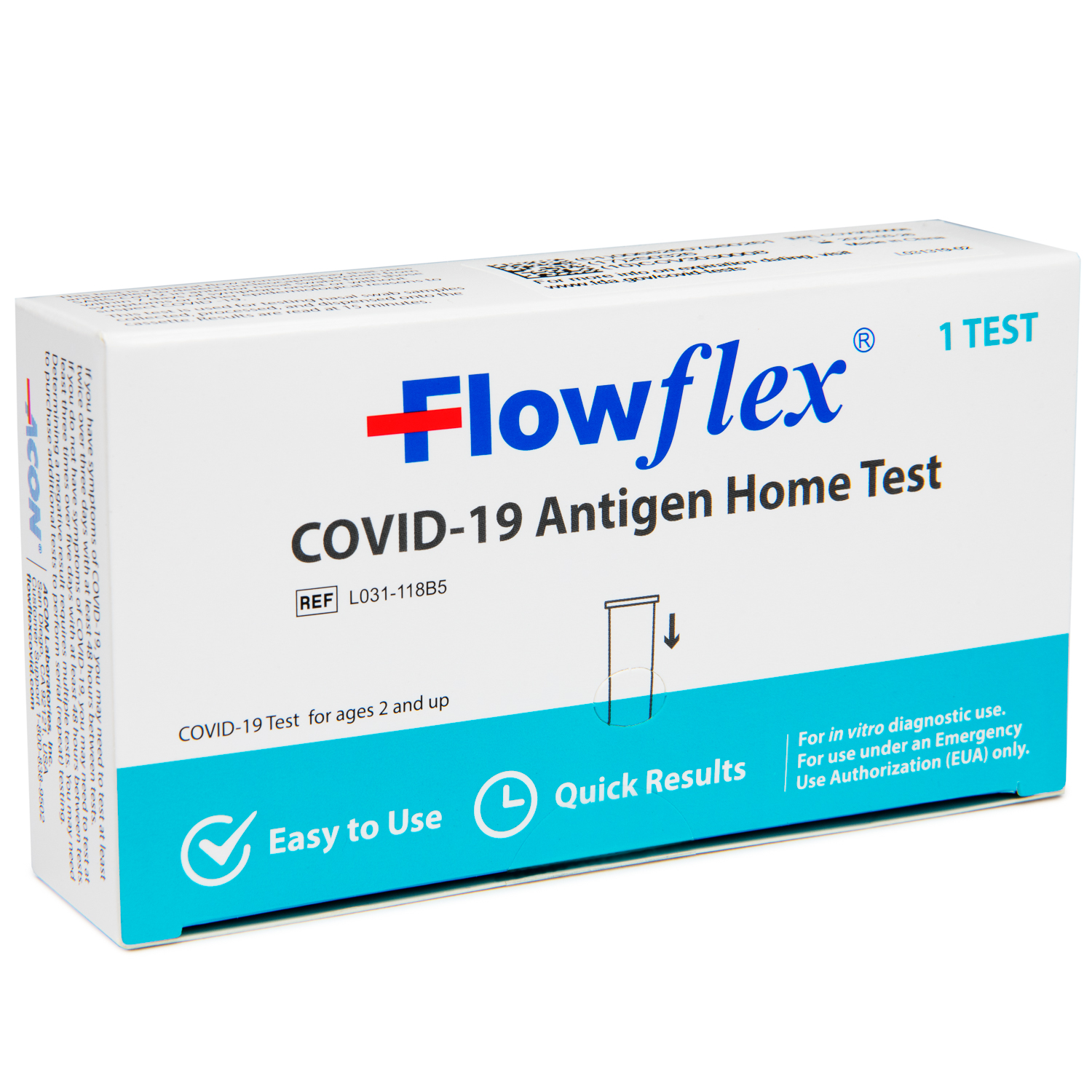
Flowflex™ Covid-19 Antigen Home Test is a highly accurate rapid test for the detection of SARS-CoV-2 antigens in anterior nasal specimens. This test is designed and approved for Self-Testing and is made affordable, so you can conveniently stay up to date on your whole Family’s health. For use under an Emergency Use Authorization (EUA) only.
Description
*FREE GROUND SHIPPING!* Simply choose “Free Ground” in checkout when the FlowFlex™ test is in your cart!
50 Tests Pack ($4.30 per test) (1 Test/Box).
The Flowflex™ COVID-19 Antigen Home Test is all you need to determine your family’s Covid-19 status, whether symptoms are present or not.
This is a rapid, reliable test for the detection of SARS-CoV-2 antigens in anterior nasal specimens.
No prescription or insurance required! This affordable, safe, at home self-test is simple to use and can be used on children as young as 2 years old.
Get the convenience of Flowflex!
- Easy and Affordable
- Highly Accurate, Easy-to-use Nasal Swab Test
- Quick Results in 15 minutes
- Safe for children as young as 2 years old
- For use with and without COVID-19 symptoms
Flowflex Covid-19 Antigen Home Test Sales Sheet
Flowflex Covid-19 Antigen Home Test Consumer Package Insert- English
Flowflex Covid-19 Antigen Home Test Consumer Package Insert- Spanish
Flowflex Covid-19 Antigen Home Test FDA EUA Letter Approval
Flowflex Covid-19 Antigen Home Test Healthcare Providers Package Insert- English
Flowflex Covid-19 Antigen Home Test Fact Sheet Healthcare Professionals
Flowflex Covid-19 Antigen Home Test FAQ
Flowflex Covid-19 Antigen Home Test Safety Data Sheet
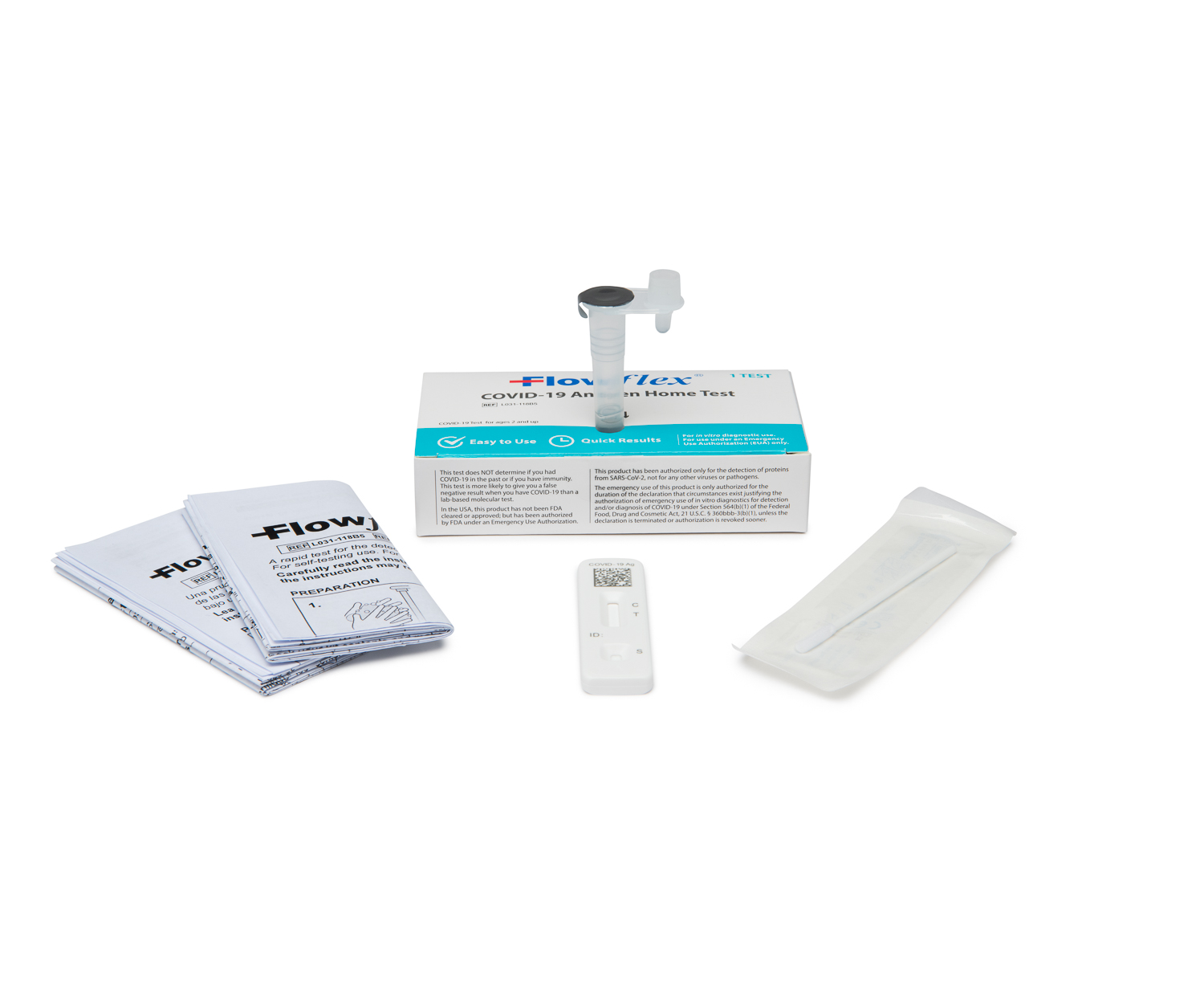
This test uses a nasal swab sample to determine the presence or absence of COVID-19 antigens in nasal samples. For a demonstration on how this test works, watch the instructional video.
- This product has not been been FDA cleared or approved; but has been authorized by FDA under an EUA; https://www.fda.gov/media/152700/download
- This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; and,
- This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.
For more information on EUAs please visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19




Letty G. –
-Letty G. Rio Grande Valley
Andrew K. –
– Andrew K. New York